The US Food and Drug Administration grants new status to Sensionics' Eversense
The US Food and Drug Administration has designated the Eversense implanted continuous glucose monitor as an"integrated CGM ," meaning it can be used in conjunction withThe Eversense now joins Dexcom's G6 and G7 and the Freestyle Libre 2 Plus in being compatible with multiple different branded insulin pumps as part of AID systems, and it is the only implantable one.
The sensor device is inserted under the skin of the patient's upper arm by a healthcare provider, and a transmitter is worn over it on the skin. TheFingerstick blood glucose measurements are still required for calibration once a day after day 21, when symptoms don't match the CGM information, or when taking
According to Sensionics, the Eversense is"the most accurate CGM in the critical low glucose ranges with essentially no compression lows." The latter refers to 'false low' alarms that sometimes occur when a person presses on the device, such as during sleep. "As we look ahead, we are focused on progressing our partnership discussions and software developments, and look forward to providing more updates," Sensionics said in a statement.
Miriam E. Tucker is a freelance journalist based in the Washington DC area. She is a regular contributor to Medscape, with other work appearing in the Washington Post, NPR's Shots blog, and Diatribe. She is on X @MiriamETucker.All material on this website is protected by copyright, Copyright © 1994-2024 by WebMD LLC. This website also contains material copyrighted by 3rd parties.
Continuous Glucose Monitoring (CGM) CGM U.S. Food And Drug Administration United States Food And Drug Administration Fda Blood Glucose Test Insulin Pump Continuous Subcutaneous Insulin Infusion Diabetes Mellitus Type 1 Diabetes Mellitus Type I Type 1 Diabetes Type 1 DM T1DM T1D Blood Sleep
Nigeria Latest News, Nigeria Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Chinese researchers engineer implantable batteries, sustained by body oxygenTwo weeks after implantation of the oxygen-run battery in rats, the researchers observed stable voltage output between 1.3-1.4 V.
Chinese researchers engineer implantable batteries, sustained by body oxygenTwo weeks after implantation of the oxygen-run battery in rats, the researchers observed stable voltage output between 1.3-1.4 V.
Read more »
 Revolutionizing Brain Health: Rice University Unveils Tiny, Implantable Brain StimulatorScience, Space and Technology News 2024
Revolutionizing Brain Health: Rice University Unveils Tiny, Implantable Brain StimulatorScience, Space and Technology News 2024
Read more »
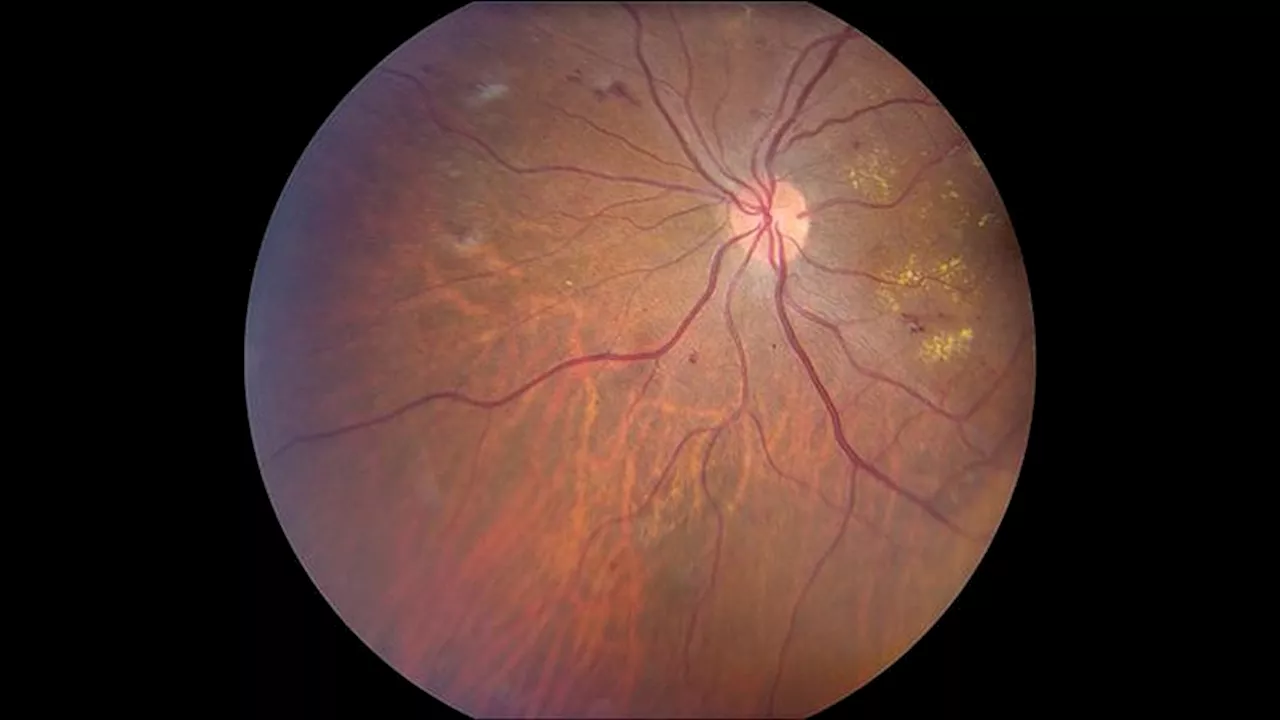 CGM Associated With Lower Retinopathy Risk in T1DThe use of continuous glucose monitoring (CGM) was linked to a reduced risk for diabetic retinopathy, a debilitating complication in adults with type 1 diabetes.
CGM Associated With Lower Retinopathy Risk in T1DThe use of continuous glucose monitoring (CGM) was linked to a reduced risk for diabetic retinopathy, a debilitating complication in adults with type 1 diabetes.
Read more »
 Use Continuous Glucose Monitoring to Diagnose Diabetes?New data showed considerable intrapersonal variability in fasting glucose and suggested that CGM adds important information.
Use Continuous Glucose Monitoring to Diagnose Diabetes?New data showed considerable intrapersonal variability in fasting glucose and suggested that CGM adds important information.
Read more »
 CPAP replacement works well for the overweight, not obese, study findsAn implantable, FDA-approved device for obstructive sleep apnea — designed to replace a CPAP — works best for people who aren’t too overweight, a new study finds.
CPAP replacement works well for the overweight, not obese, study findsAn implantable, FDA-approved device for obstructive sleep apnea — designed to replace a CPAP — works best for people who aren’t too overweight, a new study finds.
Read more »
